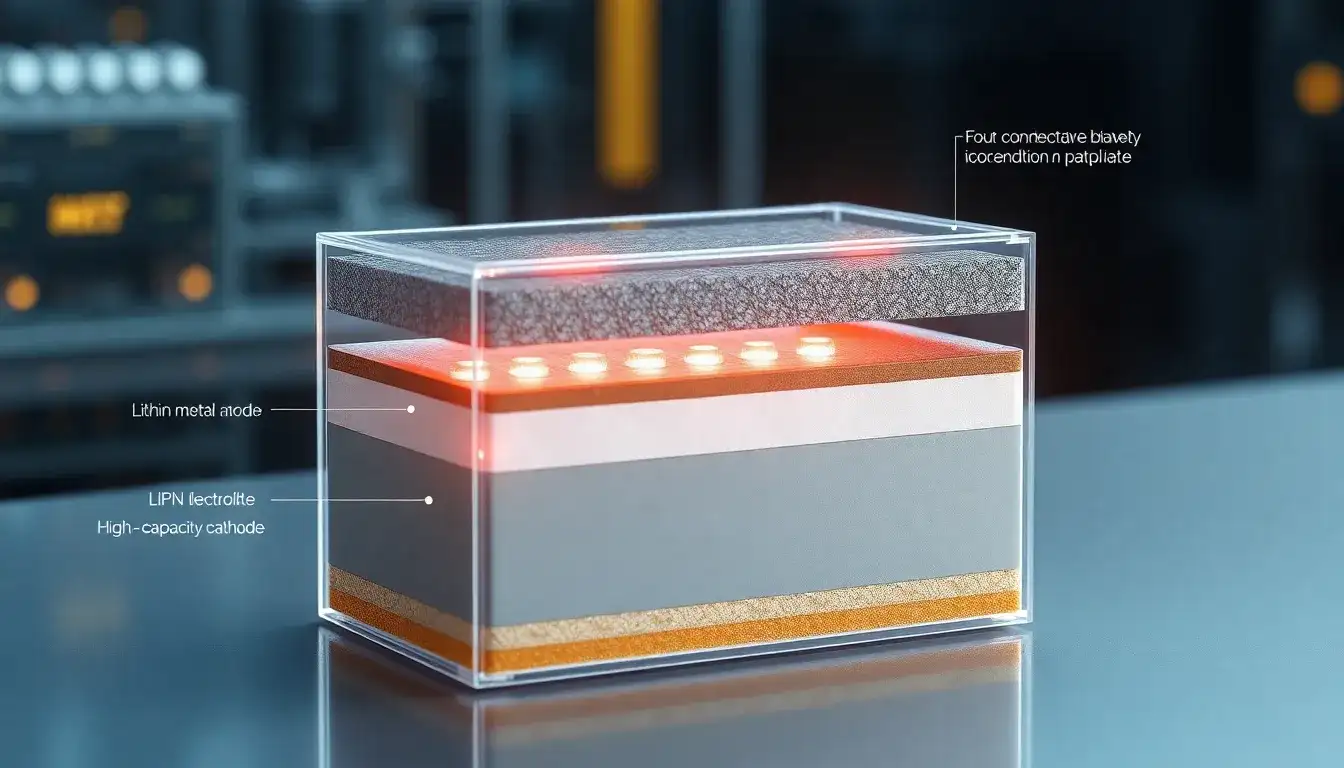
Materials Used in Solid-State Batteries
Solid-state batteries utilize a variety of materials, which can be categorized into anodes, cathodes, and electrolytes.
Anode Materials
- Metallic Lithium: Offers high energy density and is commonly used in solid-state batteries. It is especially favored for solid-state lithium-ion batteries and solid-state lithium-sulfur batteries.
- Carbon Materials: These are also used, often in the form of carbon nanotubes, due to their high specific surface area and excellent electrochemical performance.
- Silicon Materials: Utilized due to their high capacity, though they require additional design considerations to manage volume changes during charge/discharge cycles.
Cathode Materials
- Lithium Cobalt Oxide (LiCoO2): Known for high energy density and long cycle life, but poses safety risks.
- Lithium Iron Phosphate (LiFePO4): Offers better safety and longer lifespan, though with lower energy density.
- Lithium Nickel Cobalt Oxide (LiNiCoO2): Provides high energy density and long cycle life but is costly and has safety concerns.
Electrolyte Materials
- Ceramic Electrolytes (Oxides, Phosphates): Inorganic materials providing stability and safety benefits but can be challenging to manufacture due to brittleness and poor interface contact.
- Sulfide Electrolytes: Show high ionic conductivity and are considered promising for future developments, especially in all-solid-state batteries.
- Solid Polymer Electrolytes: Easy to process but limited by low ionic conductivity and chemical stability issues.
Each of these materials offers unique advantages and challenges, contributing to the ongoing development of solid-state battery technology.
Original article by NenPower, If reposted, please credit the source: https://nenpower.com/blog/what-materials-are-typically-used-in-solid-state-batteries/


