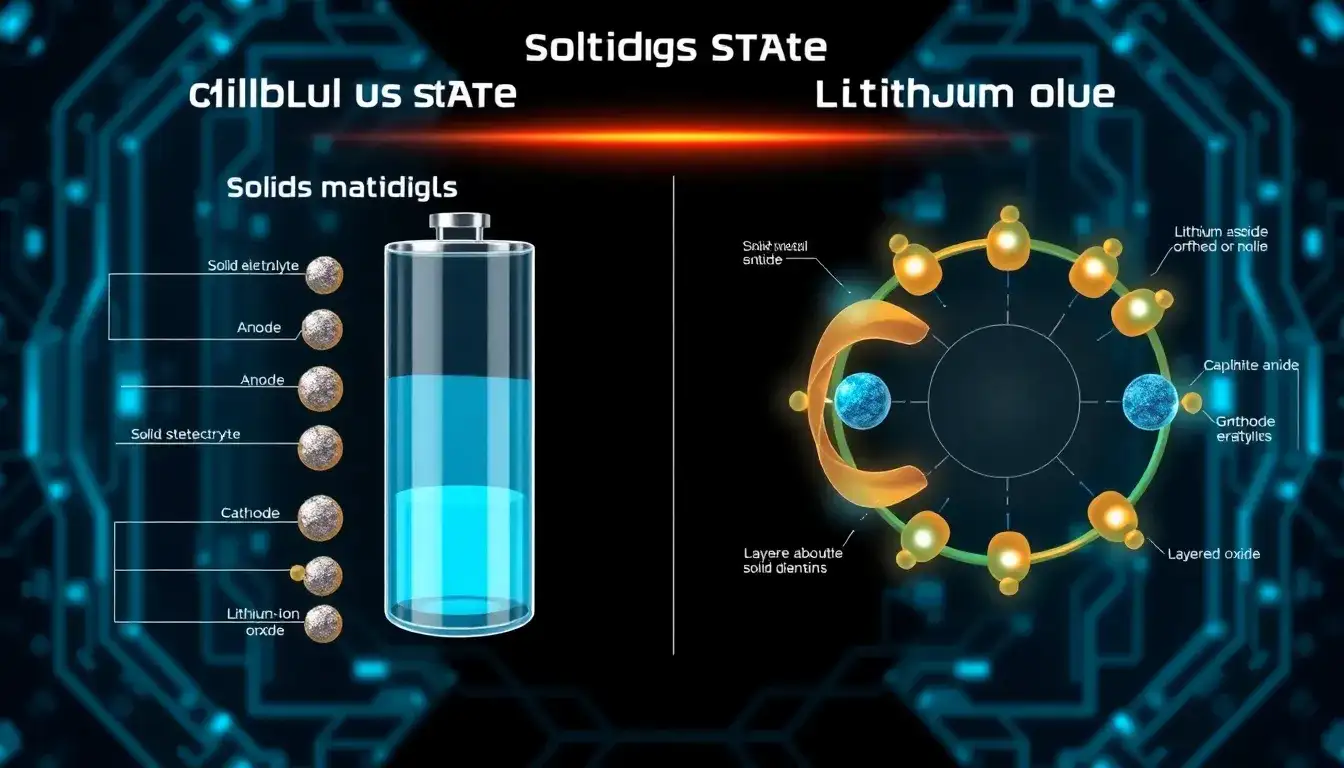
The key differences in materials between solid-state batteries (SSBs) and conventional lithium-ion batteries primarily lie in the electrolyte and anode materials, while cathode materials can have some overlap.
Electrolyte Materials
- Lithium-ion batteries typically use a liquid or gel polymer electrolyte that facilitates ion transport between the cathode and anode. These liquid electrolytes pose safety risks such as flammability and instability at higher voltages and temperatures.
- Solid-state batteries, by contrast, use a solid electrolyte which can be:
- Ceramics: including oxides, sulfides, phosphates.
- Solid polymers: organic polymers with advantages in processing but lower ionic conductivity and chemical stability.
- These solid electrolytes serve as both the ion conductor and separator, allowing only lithium ions to pass through, significantly improving safety and potentially energy density.
Anode Materials
- Lithium-ion batteries often use carbon-based anodes (graphite).
- Solid-state batteries can use metallic lithium anodes, which have much higher energy density than carbon or silicon anodes in conventional lithium-ion cells. Alternatively, carbon and silicon materials can also be used in solid-state battery anodes, but metallic lithium is a defining feature due to the solid electrolyte’s ability to suppress dendrite formation, enhancing safety and performance.
Cathode Materials
- Both battery types commonly use similar cathode materials, including:
- Lithium Cobalt Oxide (LiCoO₂)
- Lithium Iron Phosphate (LiFePO₄)
- Lithium Nickel Cobalt Oxide (NMC)
- Lithium Cobalt Aluminum Oxide (LiCoAlO₂)
- These cathode materials are stable and well-understood in lithium-ion technology and are adapted for use in solid-state designs as well.
Summary Table
| Battery Component | Lithium-ion Battery Materials | Solid-state Battery Materials |
|---|---|---|
| Electrolyte | Liquid or gel polymer electrolytes | Solid ceramics (oxides, sulfides, phosphates), solid polymers |
| Anode | Carbon (graphite), silicon | Metallic lithium, carbon, silicon |
| Cathode | Lithium cobalt oxide, NMC, LFP, etc. | Lithium cobalt oxide, NMC, LFP, etc. |
Implications
- Solid electrolytes enable the use of metallic lithium anodes, potentially increasing energy density and safety by eliminating flammable liquid electrolytes.
- Trade-offs exist in solid electrolytes; polymer solid electrolytes have easier processing but lower conductivity and stability, while ceramic electrolytes are more stable but can be brittle and expensive.
In essence, the main material difference is that solid-state batteries replace the liquid electrolyte with a solid electrolyte and often use metallic lithium anodes, whereas lithium-ion batteries rely on liquid electrolytes and carbon-based anodes.
Original article by NenPower, If reposted, please credit the source: https://nenpower.com/blog/what-are-the-differences-in-the-materials-used-for-solid-state-batteries-compared-to-lithium-ion-batteries/


